Safety and efficacy of hydroxyurea (HU)
Rationale
HU is the only drug currently approved by the US FDA for use in SCD to reduce risk of clinical events. Presumably through its ability to increase levels of HbF, clinical trials have overwhelmingly supported the efficacy of this agent in both adults and children. In the US, this agent is reserved for high-risk patients – generally about 30-40% of clinical cohorts. A recent trial in Brazil – which has not yet been published – demonstrated improved survival among patients receiving HU.
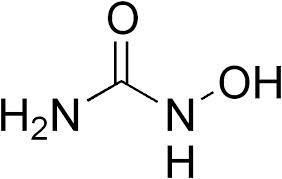
HU suppresses hematopoiesis and requires close monitoring; in addition, the concern exists about increased susceptibility to infection, which would be a major hazard in Africa. In addition to expense and availability, this drug has therefore not been widely used in Africa.
Substantial research will be required to demonstrate safety, efficacy and the optimal dose regimen for resource poor settings in Africa. Three small-scale trials are now underway in Nigeria and Tanzania and may provide some useful preliminary information about hematologic outcomes with HU. (African trials of HU are likely to share the low-resource conditions being reported from India with fixed low-dose HU, but will differ in patterns of infectious disease and differ in genetic “modulators” from sickle cell disease in India. A large-scale, definitive trial will require substantial resources, including improvement of the infrastructure at the participating clinical centers. A working group should be established to formulate a plan as to how to best move forward toward use of HU in Africa and other low-income settings.
